FOR GOODS,COSME,
QUASI-DRUG
Globule SW-20
[ Globule® ]

Application
- Soaps / Detergent
- Cosmetics
- Sprays
- Deodorant for hospital care
- Toiletry goods
Source of odor
- Body odor
- Generational odor
- Fecal odor
The main component is 1,8-cineole contained in eucalyptus essential oil, and it deodorizes odorous components contained in cancer odor, body odor and fecal odor by chemical reaction.
Deodorant Performance
- Ammonia
- Trimethylamine
- Acetic acid
- Isovaleric acid
- n-Butyric acid
- Nonenal
- Diacetyl
- Pelargonic acid
- Skatole
Product Information
| Base | This product is a highly safe water-soluble raw deodorizing material prepared by mixing essential oils and organic acids—obtained from the leaves of Eucalyptus globulus Labillardiere and other closely related plants (Myrtaceae) through steam distillation—with surfactant, water, and other ingredients. |
|---|---|
| Features |
It is a highly safe deodorant composed only of raw materials that meet the Japanese Standards of Quasi-drug Ingredients2021, including "eucalyptus oil" that contains a high content of the active ingredient 1,8 cineole. The eucalyptol combines with highly hydrophobic malodorous components, which initiates chemical reactions such as Van Der Waals force or hydrogen bonding to reduce the concentration of the malodorous component in a given space. |
| Characteristics |
① Appearance ・・・ Colorless to slightly yellow transparent liquid ② Odor ・・・ Eucalyptus-like scent ③ pH ・・・ 3.5 to 5.5(20℃) ④ Specific Gravity ・・・ 0.900 to 1.100(20°C) ⑤ Solubility ・・・ Easily soluble in water and ethanol |
| Application | Miscellaneous goods, cosmetics, quasi-drug products (composed of raw materials listed in the Japanese Standards of Quasi-drug Ingredients 2021) |
Antiseptic/Antifungal Performance Test
Antiseptic/antifungal effects have been confirmed in aqueous solutions of at least 0.5%.
| Sample | Globule® SW-20 (0.5% aqueous solution) Globule SW-20 (1.0% aqueous solution) |
|---|---|
| Experiment Method | Preservatives-Effectiveness Test (Japanese Pharmacopoeia 17th Edition) |
| Test bacteria | Escherichia coli(E.c) NBRC 3972 Pseudomonas aeruginosa(P.a) NBRC 13275 Staphylococcus aureus(S.a) NBRC 13276 Candida albicans(C.a) NBRC 1594 Aspergillus brasiliensis(A.b) NBRC 9455 |
| Inoculation and Storage | 20 g of the sample for each test bacterium isplaced in a sterile vial and inoculated with 0.15 mL of the test bacterium solution (105-106 per 1 g of the sample). Each vial is stored at 22.5°C, and the viable cell count is measuredon the 7th, 14th, 21st, and 28th day. |
| Viable Cell Count Measurement | SCDLP Agar Medium Pouring for bacteria, and Sabouraud Dextrose Agar with Lecithinand Polysorbate Medium Pouring for fungi(yeast, mold). |
| Test Results |
Changes in sample bacteria count afterinoculation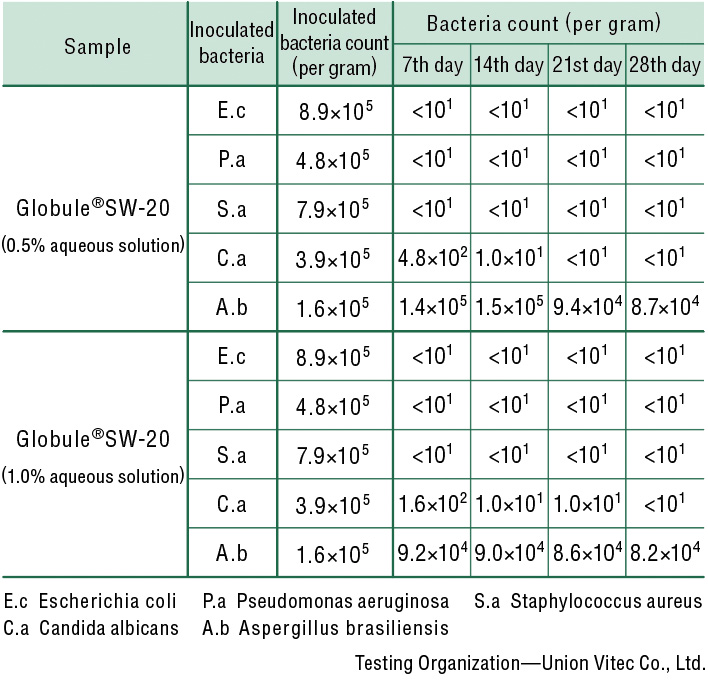
|
If there are any other products you are looking for,
please feel free to contact us.
If there are any concern other than the product, please do not hesitate to contact us.
We will respond sincerely to all questions and consultations until your problem is resolved.